Shyam was only 31 when his heart stopped beating in November 2018.
A property broker and former high school volleyball player, Shyam had recently gained weight. During a workout one day, he felt short of breath and insisted that friends rush him to hospital. Minutes later, his pulse flat-lined.
He survived the heart attack, but the scar tissue that resulted cut his heart’s pumping ability by a third. He couldn’t pick up his children. He fell asleep every night wondering if he would wake up in the morning.
Desperation motivated Shyam to try for an unusual medical treatment: getting stem cells injected directly into his heart.
“I just trusted my doctors and the science behind it, and said, ‘This is my only chance,’” he recalled.
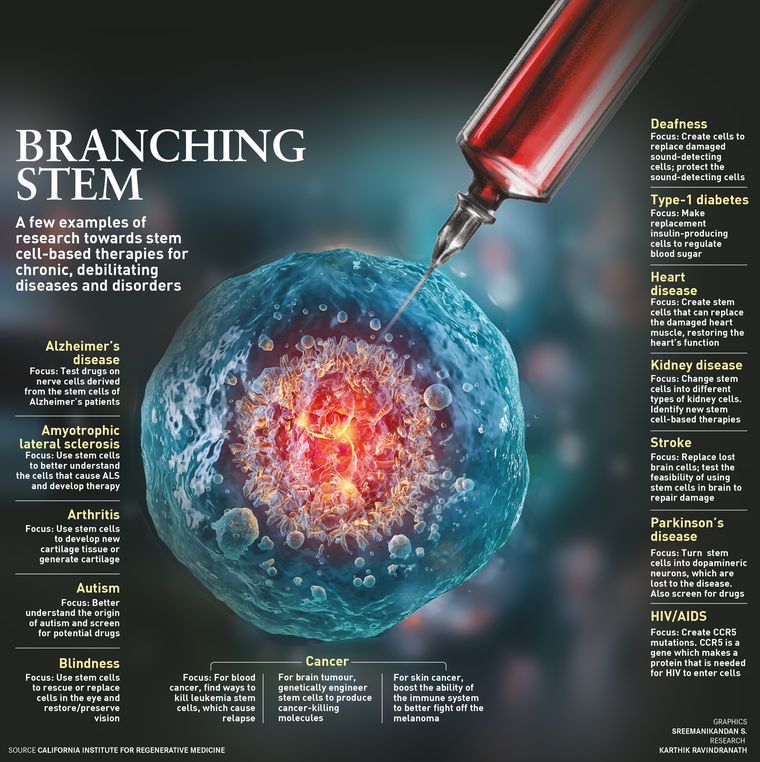
Over the last decade, by studying stem cells in lab dishes, test animals and patients like Shyam, researchers have brought the promise of stem cell therapies closer to reality. The use of stem cells is part of a field called regenerative medicine, wherein a body’s own cells and growth factors are deployed to repair tissues by restoring their lost function. Several cellular therapies and products have already been approved by regulators and are in use, including skin substitutes for treating burns, ‘scaffold’ products for healing surgical incisions and products derived from umbilical cord blood for treating certain blood diseases and disorders.
Stem cells broke into the public consciousness in the early 1990s, alluring for their potential to help the body beat back diseases of degeneration like Alzheimer’s, and to grow new parts to treat conditions like spinal cord injuries. Progress has been slow. But researchers have been persistently learning how to best use stem cells, what types to use and how to deliver them to the body―findings that are not singularly transformational, but progressive and pragmatic.
As many as 6,000 clinical trials involving stem cells are underway in the US to treat patients with heart disease, blindness, Parkinson’s, HIV, diabetes, blood cancers and spinal cord injuries, among other conditions. Initial studies suggest that stem cell therapy can be delivered safely. However, hurdles include producing consistent, high-quality therapies, receiving federal approval and persuading insurers to cover the treatments.
Stem cells harvested from an embryo can turn into any of the body’s 200 cell types and, theoretically, live as long as the body does, unlike most cells. The basic idea of therapies using stem cells is simple: inject them, for example, into a brain whose cells are dying, and the replacement cells could presumably grow. The same would hold true for muscles, blood, organs and bone. In theory, stem cells can make repairs, lead to new growth and replace missing pieces.
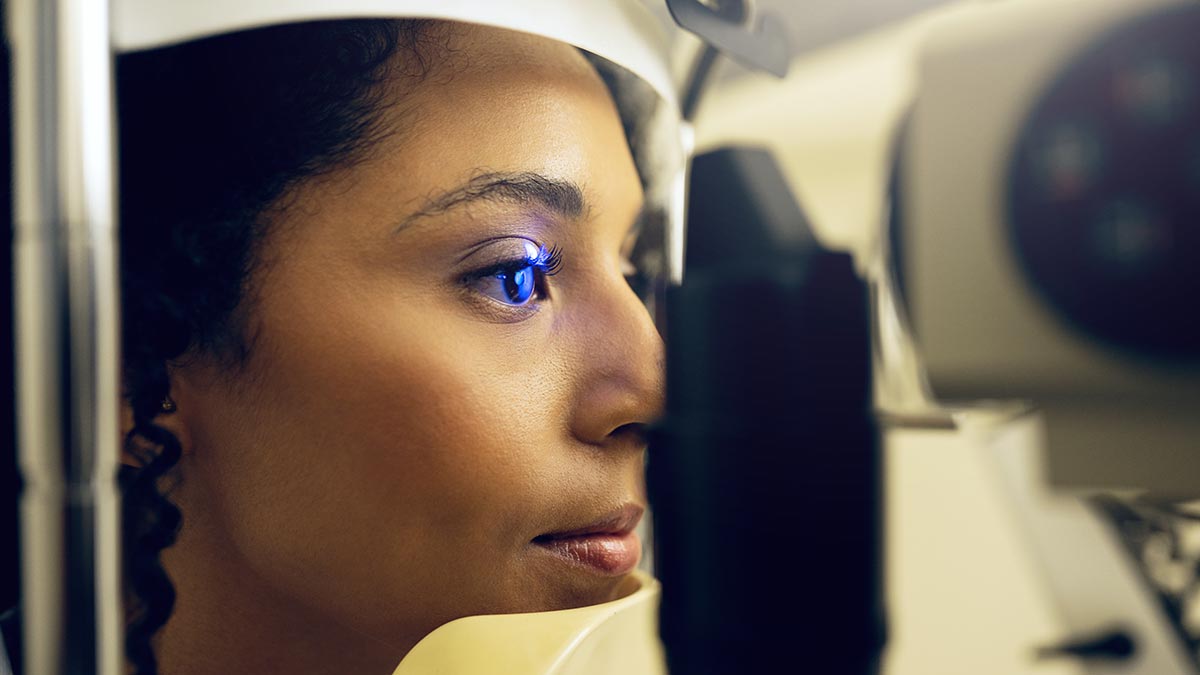 Future vision: Apart from treating conditions like macular degeneration, stem cell treatments have been applied to repair damaged corneas in patients with severe eye injuries, restoring vision and enhancing the healing process | Shutterstock
Future vision: Apart from treating conditions like macular degeneration, stem cell treatments have been applied to repair damaged corneas in patients with severe eye injuries, restoring vision and enhancing the healing process | Shutterstock
“When we think about stem cell therapy, we often think of situations where we give a cell that can give rise to other cells and repopulate and continue to maintain a person hopefully for the rest of their lives,” explains Dr Lawrence Fong, cancer immunotherapist at UCSF Helen Diller Family Comprehensive Cancer Center on CureTalks. “And the best example of that is the bone marrow transplants or what we also call peripheral stem cell therapies, where we can actually harvest circulating stem cells from a patient or from a donor and then give those back to a patient. And those stem cells are actually able to repopulate, in this case, a patient's immune system. We are looking at other tissues, too, because as we have learned in the laboratory, there are stem cells for many different types of tissues. This is an active area of research in terms of trying to see what stem cells we could give to a patient that might restore a tissue impacted by disease.”
Understanding how stem cells work requires some basic biology
“A stem cell is a kind of cellular promise―an embryonic wish, a hopeful origin story―from which a whole organism can grow,” writes Dr Siddhartha Mukherjee in The Song of the Cell: An Exploration of Medicine and the New Human.
 Heart matters: Scientists presumed that a patient’s heart would repair itself better when injected with its own stem cells. But a study led by Joshua Hare at the University of Miami showed that patients fared just as well with someone else’s stem cells | Shutterstock
Heart matters: Scientists presumed that a patient’s heart would repair itself better when injected with its own stem cells. But a study led by Joshua Hare at the University of Miami showed that patients fared just as well with someone else’s stem cells | Shutterstock
Stem cells are the only cells in your body that can make different kinds of cells. Some stem cells can multiply or make more of themselves or other types of specialised cells that make up your blood, brain, muscles, bones and more. Other stem cells have less potential for self-renewal and cannot make as many types of cells.
Every cell in the body has the same set of DNA, although different genetic material may be active in, say, a nerve cell and a blood cell. Embryonic stem cells, derived from the first cells created after conception, can live as long as the body does, with the potential to make every other cell type in the body.
 Bone hone: In the field of orthopaedics, stem cell therapy has shown success in treating conditions like osteoarthritis, bone fractures and non-union injuries | Shutterstock
Bone hone: In the field of orthopaedics, stem cell therapy has shown success in treating conditions like osteoarthritis, bone fractures and non-union injuries | Shutterstock
If the embryonic stem cell is at the top of every cell’s family tree, the first branches are different kinds of stem cells, like those that give rise to all blood, muscle or brain cells. Below those are even more restricted precursor cells―parents of all heart cells, for example.
An early breakthrough occurred in 2006, when Shinya Yamanaka, a molecular biologist at Kyoto University in Japan, showed that stem cell therapy could avoid the morally divisive use of embryonic stem cells. Instead, he discovered adult skin cells could be induced to develop into blood, bone or liver cells, just as the stem cells of a human embryo evolve into various parts of the body. Yamanaka, who went on to win the Nobel Prize in 2012 for this work, called these “induced pluripotent stem cells” or iPSCs. This discovery moved the stem cell conversation past the sensitive subject of using foetal tissue for medical purposes, which is illegal in some countries.
 Dr Lawrence Fong
Dr Lawrence Fong
Yamanaka’s cocktail allows researchers to, say, turn a skin cell back into an iPSC. Now, researchers can move the reverted cell forward, too, making, say, a precursor cell to inject into a beating heart.
But figuring out which type of cell best addresses a particular medical condition remains a major research challenge. For instance, injecting embryonic stem cells into a patient might solve the problem, might do nothing, or might seed a tumour called a teratoma. And it may be years before the outcome is obvious, as in the case of a paraplegic woman in America, who had stem cells from her nose implanted into her spine as part of a clinical trial in Portugal. The therapy failed, and eight years later, the woman had a tumour-like mass of nasal tissue surgically removed from the implant site.
Beyond direct therapies, stem cells are also giving researchers new tools in the lab. Using cells created from patients with specific ailments, it is possible to reproduce and study diseases in a dish.
So, what is state of the art in this exciting field?
Tokyo’s Riken Research Institute performed the first successful iPSC transplant in 2014, creating retinal cells generated from skin cells of a patient with age-related macular degeneration, a serious eye condition. Shortly afterwards, Dr Henry Klassen at the University of California, Irvine, oversaw a trial for treating retinitis pigmentosa, a group of rare genetic disorders causing gradual blindness, in which a donor’s retinal progenitor cells were transplanted into the eyes of 28 study participants. One of them, 64-year-old Kristin Macdonald of Los Angeles, who had gone blind, regained what she describes as a burst of light. “I can navigate by light now, and see more contrasts and shapes,” she says. Macdonald has since become an outspoken patient advocate for stem cell trials.
Then, in 2018, neurosurgeon Richard Fessler of Rush University Medical Center in Chicago oversaw a year-long trial with iPSC-derived motor neurons transplanted into six patients paralysed by spinal cord injuries. Fessler reported that all regained some upper body movement and that a patient who was only able to shrug his shoulders could now use his hands to eat, write and do other tasks.
This is the kind of breakthrough that the late actor Christopher Reeve, best known for playing Superman, was advocating for from his wheelchair after suffering neck-down paralysis following a horseback-riding accident. At that time, governments around the world were making moves to restrict embryonic stem cell research. (In certain cases, embryonic stem cells are in medical use today; they are derived from leftover blastocysts―the clustering of cells in a fertilised egg―that didn’t implant during IVF treatment.)
These are still early days, with much to investigate in terms of safety, dosing and how to manufacture iPSCs for different conditions in a standardised and cost-effective way. But Yamanaka predicted in 2018 that several treatments using regenerative medicine and new drugs will be developed and authorised by around 2030.
In the meantime, the first generation of stem cell treatments that have regulatory approval in Europe, Canada and the US largely involve simple cell transplants―ones that move the patient’s own cells from one part of their body to another. That said, stem cell transplants for blood diseases like leukaemia, which have been carried out for several years, are the one exception where cells from a matching donor are also allowed.
Transforming treatments, lives
In a recent groundbreaking procedure, Chinese scientists have reportedly cured a 25-year-old woman with type 1 diabetes using a novel stem cell transplant. The patient, who had been managing her condition for over a decade, underwent a minimally invasive surgery that lasted just half an hour. Approximately two and a half months post procedure, she began naturally regulating her blood sugar levels without the need for insulin injections. At the one-year mark, she still had no need for insulin injections. This significant advancement, detailed in the journal Cell, involved reprogramming the patient's own adipose (fat) tissue cells into pluripotent stem cells using small molecule chemicals. These reprogrammed cells were then transformed into islet cells, responsible for insulin production, and transplanted back into her body. The success of this approach offers promising prospects for new diabetes treatments.
Type 1 diabetes is an autoimmune condition that results in the destruction of the islet cells by the immune system. This means that the body cannot create as much insulin as needed, which results in chronic, high blood glucose that can lead to complications like eyesight issues and nerve and kidney damage. The patient had previously had two liver transplants and a failed pancreas transplant due to complications that had risen due to her diabetes. The induced islet cells made from the patient’s own cells were injected between the skin and abdominal muscles.
Though this is the first case study available of a person who has continued to produce insulin one year after receiving stem cell-based therapies, there are other trials that are ongoing to develop a stem cell treatment for people with type 1 and 2 diabetes.
Over the past few decades, researchers have explored the application of stem cell therapies across numerous therapeutic areas, with several achieving varying degrees of significant success. One of the most successful and longstanding uses of stem cell therapy has been in treating haematological disorders. Conditions like leukaemia, lymphoma, and sickle cell anaemia have seen significant advancements through hematopoietic stem cell transplantation (HSCT), also known as bone marrow transplantation. This therapy replaces diseased or damaged bone marrow with healthy stem cells, allowing the regeneration of blood and immune systems.
Another therapeutic area where stem cells are showing great promise is in the treatment of neurological disorders. Parkinson’s disease (PD) is a chronic neurodegenerative disorder for which disease-modifying therapy or neuroprotective techniques are not available at the moment. The current treatment modalities are limited to control of symptoms with medications which comes with its own set of complications of motor fluctuations and uncontrolled muscle movement. Deep brain stimulation (DBS) is another effective but invasive option for symptom control.
Dr Preetha Menon, consultant, division of geriatric medicine, department of medicine, Alexandra Hospital, Singapore, shares, “There are several phase I and II trials for its consideration as an alternative to levodopa or DBS as these cells have the potential to replace diseased neurons or degenerated tissues. Among the various types of stem cell therapies out there, mesenchymal stem cells (MSC) and iPSC are the most promising for PD. MSCs have the advantage of being derived from the same patients, increasing the potential for individualised therapy without triggering immunological reactions. The different modalities of transplantation being explored are inclusive of direct implantation into key areas of the brain, intravenous, intraarterial, intramuscular and even intranasal routes. The biggest challenge with cell-based therapy, in addition to immune response and tumorigenesis (tumour development), is that therapeutic benefits may not be sustained for a satisfactory period of time. Regardless, it is likely to be at the forefront of research towards discovering a disease-modifying treatment in the future and is an exciting road ahead.”
In the field of orthopaedics, stem cell therapy has shown success in treating conditions like osteoarthritis, bone fractures and non-union injuries. For patients suffering from chronic joint pain, stem cell injections have been effective in regenerating cartilage, reducing inflammation and improving mobility. Similarly, bone healing has been enhanced in cases where traditional methods had previously failed, providing a less invasive and more effective solution. Autoimmune diseases, including rheumatoid arthritis and lupus, have also been targeted using stem cell therapy. MSCs have shown the ability to reduce inflammation and slow disease progression. Patients with lupus, for instance, have reported significant improvements in symptoms, including better kidney function and reduced flare-ups, after undergoing stem cell treatment. Stem cell therapy is making significant strides in ophthalmology, too. Apart from treating conditions like macular degeneration, stem cell treatments have been applied to repair damaged corneas in patients with severe eye injuries, restoring vision and enhancing the healing process.
Vivek was a 29-year-old physics PhD student when his life took an unexpected turn. One day, while lifting a heavy instrument at work, he felt a sudden, intense pain in his back. The diagnosis? Three-level degenerative disc disease―a condition where the discs between the vertebrae in his spine were worn out and causing severe pain.
To confirm the problem, doctors performed a discogram, a procedure that pinpointed which discs were causing the pain. It turned out all three damaged discs were contributing. Vivek had surgery to replace two of the worst discs, which helped a little, but not enough. He was still dealing with constant, sharp pain in his lower back that spread to both legs, including his thighs, calves and even his feet. He described the pain as a “sharp dagger-like sensation” along with a deep ache. His legs felt like they were burning and bruised, making it almost impossible to move around comfortably.
Vivek’s life changed drastically. Most of his days were spent in bed. He couldn’t walk far, sit for long, or even drive without being in pain. Simple tasks like doing laundry or walking the dog became almost impossible.
In October 2015, Vivek decided to try stem cell therapy. Doctors injected stem cells into his L3-4 disc, one of the damaged areas in his spine. Just five weeks after the procedure, the pain in his lower back, which used to cover a large area, was now confined to a small spot about three inches wide. Even more impressive, the pain in his legs almost completely disappeared. He occasionally felt a small twinge in his left buttock, but that was it. While Vivek still experienced some sharp back pain from time to time, it was far less frequent. The constant ache and stiffness in his back also improved. More important, his ability to live a normal life returned.
He could finally sleep better, which made a huge difference in how he felt day-to-day. He was able to do household chores again, like laundry, and even took his dog on walks several times a day. Socialising and driving, activities that had once been too painful, became part of his routine again. Overall, Vivek experienced a 90 per cent reduction in his leg pain and a 60-70 per cent improvement in his back pain. “I have got my life back,” says Vivek.
But the most cost-effective way to deliver stem cells is yet to be determined. Scientists presumed, for instance, that a patient’s heart would repair itself better when injected with its own stem cells. But a study led by Joshua Hare at the University of Miami showed that patients fared just as well with someone else’s stem cells, and their bodies didn’t mount an immune attack against the cells. If supported by further studies, this means that future patients won’t need immunosuppressants, and that stem cells can be made in large batches―and therefore more cheaply.
Because regenerative medicine is still young, patients should be wary of fraudulent stem cell products being sold by unscrupulous companies. They advertise cures for everything from hair loss to Lyme disease, sometimes offering stem cells in vials, as if they were magic potions. There is reckless administration of unapproved products by non-specialists in disease, which has led to lawsuits and regulatory crackdowns.
Priya Menon is host and producer of CureTalks, and VP, TrialX.