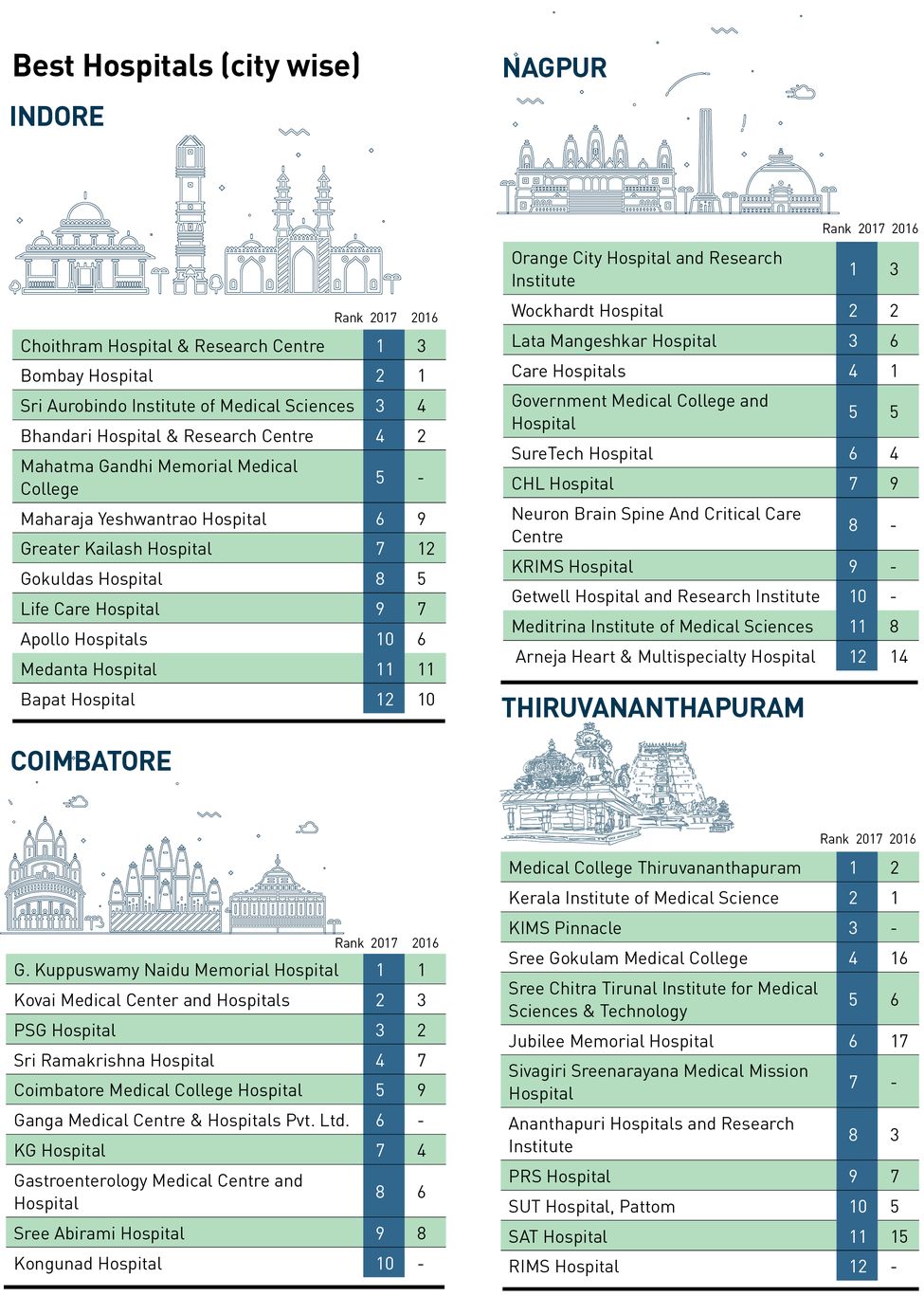
Living in despair and discomfort had become a daily affair for Smitha Jha—the ovarian cancer that was first diagnosed in 2015 had made a comeback, and there was fluid accumulation in her abdomen. The 40-year-old Mumbai resident had stopped responding to routine chemotherapy after 12 sessions and was on the verge of giving up. That’s when doctors suggested an alternative treatment called Pressurised Intraperitoneal Aerosolised Chemotherapy (PIPAC).
Dr Ninad Katdare, surgical oncologist, S.L. Raheja Hospital in Mumbai, said, “Jha had a form of ovarian cancer called mucinous adenocarcinoma, which is traditionally more resistant to routine chemotherapy,” he said. “Wherever it is operable, these patients get treated using cytoreduction surgery (CRS) with HIPEC [hyperthermic intraperitoneal chemotherapy]. However, this case was advanced even for that procedure. She was an ideal candidate for PIPAC. She responded to the treatment as the ascites or fluid in the abdomen had shrunk from 3 litres to 100ml and due to PIPAC, the new formation of ascites is also under control.” Jha was the fourth patient to have undergone the first cycle of this treatment. Only two centres in India perform the PIPAC procedure, according to Katdare. “If the patient responds to PIPAC, they can be considered for cytoreduction with HIPEC. This has been proved in a clinical trial now. In other cases, it can help in the control of disease and/or symptoms,” he said.
For patients suffering from peritoneal cancers, which mainly affect the lining of the stomach, abdominal cavity and other organs like the ovaries, gallbladder, pancreas and colon, PIPAC offers hope as it is a combination of both chemotherapy and surgery. A laparoscopic or keyhole surgery is used to directly deliver the chemotherapy drugs, with the help of carbon dioxide, to the affected areas. A special device known as a CapnoPen is used to make the incision, and after the chemotherapy, drugs are sprayed directly to the affected areas. A filter is used to absorb any residual fumes before the incision is closed. Katdare is the first Indian doctor to perform the PIPAC procedure after undergoing training at Germany’s University Hospital of Tübingen. “Of the four patients who have undergone the first cycle of PIPAC, the second patient unfortunately succumbed to ovarian cancer before the next cycle as the disease was advanced,” he said.
PIPAC costs Rs 2.5 lakh per cycle, and the treatment requires at least three cycles. However, the good news for peritoneal cancer patients is that steps are being taken to manufacture the CapnoPens, which is the most important and costly part of PIPAC, in India. “By mid-2018, the cost will come down to Rs 1 lakh per cycle. The side-effects of routine [intravenous or oral] chemotherapy like hair loss, vomiting and rashes are not seen with PIPAC. However, as PIPAC is done under general anaesthesia, the risks related to it remain,” said Katdare.
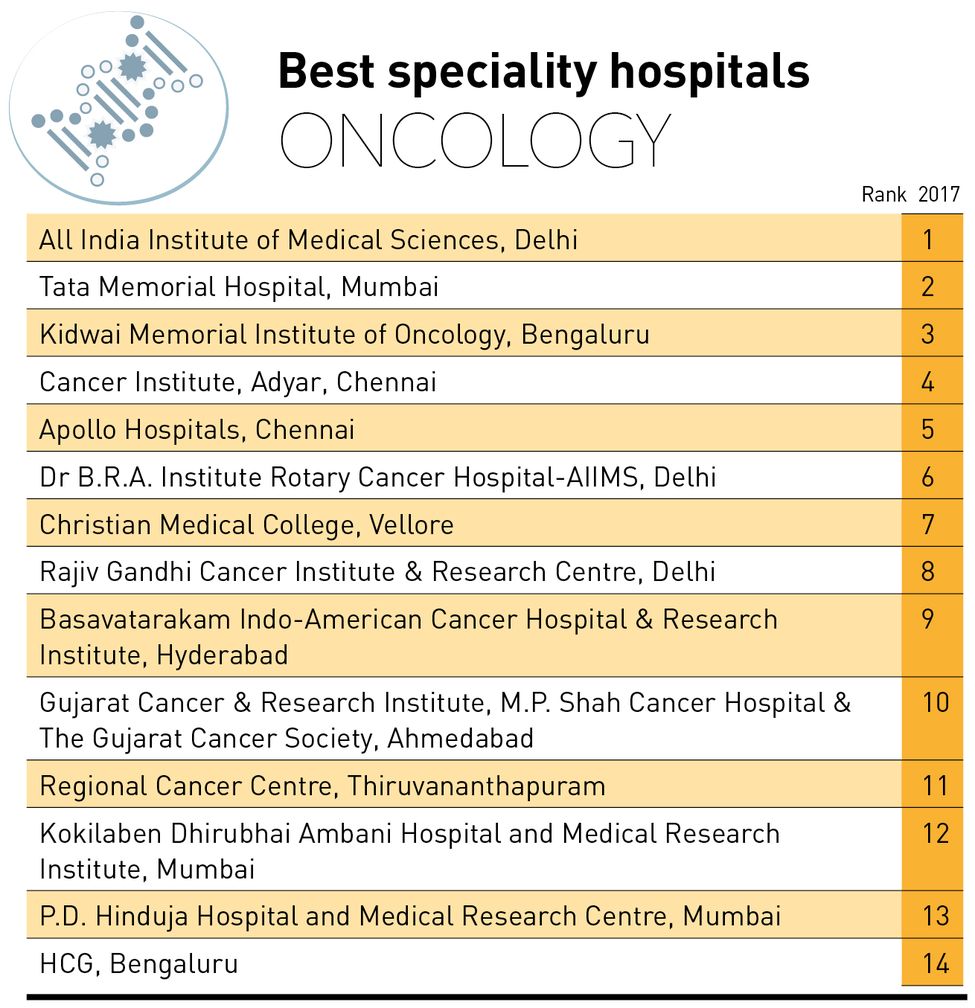
While PIPAC was invented by Professor M.A. Reymond of University of Tübingen, Indian researchers are currently working hard to develop a therapeutic vaccine, which has the potential to fight cervical and breast cancer. One lakh women die of cervical cancer every year in India, making it the second leading cause of cancer deaths among women. Breast cancer, however, tops the mortality list here, claiming nearly 1.7 lakh patients every year. Researchers at New Delhi’s National Institute of Immunology (NII), however, have extracted a tumour antigen known as SPAG9, which, they said, promises to kill tumours with no side-effects or toxicity effects.
 In search for cure: Dr Ninad Katdare of S.L. Raheja Hospital, Mumbai, is the first oncosurgeon in India to use the PIPAC procedure | Amey Mansabdar
In search for cure: Dr Ninad Katdare of S.L. Raheja Hospital, Mumbai, is the first oncosurgeon in India to use the PIPAC procedure | Amey Mansabdar
“SPAG9 antigen is involved in cancer progression. In our human clinical trials, one of the inclusion criteria of cervical cancer patients is that their tumour must be expressing SPAG9 antigen,” said Dr Anil Kumar Suri, director, NII. “Therefore, it is mandatory that only those patients whose tumour is expressing SPAG9 are included in the trial. From such patients, SPAG9 primed dendritic cells are prepared and are injected back into patients’ own dendritic cells. These dendritic cells educate the T cells to kill SPAG9 expressing tumour cells. This is how a tumour is killed specifically, with no side-effects and no toxicity effects.”
Dendritic cells are our body’s potent detector of foreign invaders such as bacteria and viruses and are also the internal police to detect if our cells are turning cancerous. “Once cancer develops, the immune system is suppressed to allow the growth of cancer. Among the factors which lead to the immune system losing its potency is suppression of the dendritic cell function in the tumour area,” said Suri.
Researchers said the immune system can be reactivated by developing dendritic cells derived from the patient’s own blood cells and then priming them with cancer antigens, before returning the mature primed dendritic cells back to the patient.
In the phase 1 of the clinical trial held between 2004 and 2006, the therapeutic vaccine was tested on 54 patients at the molecular oncology department of Cancer Institute, Adyar, Chennai. A team, led by Dr T. Rajkumar, is currently conducting the Phase 2 trial on 23 cervical cancer ( stage IIIB) patients.
“This is a three-arm double-blind randomised trial, wherein one group of patients will receive only concurrent chemotherapy with radiation and saline placebo,” said Rajkumar. “Arm 2 patients will, in addition to concurrent chemotherapy with radiation, receive dendritic cells primed with the patient’s own tumour proteins. Arm 3 patients will, in addition to chemotherapy with radiation, receive dendritic cells primed with SPAG9 protein. The number of doses of the vaccine would be ten.” The results will be out only next year. “Three patients have completed all the ten doses and will undergo evaluation next month. So far, no patient has developed toxicity due to the vaccine,” said Rajkumar.
In Mumbai, another team of researchers is gaining momentum in treating surface tumours like oral and cervical cancer with the help of gold nanoparticles. A team of four researchers from Tata Memorial Centre and Indian Institute of Technology (IIT) Bombay have developed hybrid polymer-gold nanoparticles as photothermal agents to get rid of tumours.
Dr Abhijit De from the Molecular Functional Imaging Lab at ACTREC, Tata Memorial Centre, said a thermoresponsive polymer nanoshell, which consists of an anti-cancer drug, is coated with gold nanoparticles. “The gold particles are between 3 and 5 nanometres in size, and as the kidneys have the ability to filter out anything which is below 15 nanometres, any residues or potential toxicity which could be caused by these particles is not an issue,” said De. “When the core is heated with a laser for three to five minutes after it is injected in a tumour, we have observed that the tumour completely disappears after three days. In some cases, thermal ablation is required the next day, but the results are very promising.”
Cancer patients, said De, will be able to get treated in the outpatient department of hospitals in 45 minutes through this procedure. Also, deep-seated tumours could be effectively treated with photoablation using a catheter.
Dr Rohit Srivastava, department of biosciences and bioengineering, IIT Bombay, said they are now planning on conducting the Phase I clinical trials on patients with oral cancer. “We are in the process of setting up a company in Bengaluru known as C-Camp on the NCBS [National Centre for Biological Sciences] campus,” said Srivastava, “and raise the funds required for the clinical trials through crowdfunding.”